On November 17, 2021, Simcere Pharmaceutical Group Limited (2096.HK) announced that the company has reached collaboration agreement with the Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences (CAS), to develop novel antiviral drugs for COVID-19. During preclinical studies, the compound candidates for these drugs have been shown to have strong inhibitory effects on a variety of SARS-CoV-2 strains, including the Delta variant, indicating their potential of becoming a new generation of oral drugs to treat the disease.
Under the arrangement, Simcere will obtain the exclusive right to develop, manufacture and commercialize the novel antiviral drug SIM0417 globally. Mutually striving to further contribute to the fight against the COVID-19 pandemic, both parties committed to working together to promote the clinical research and marketing process of the candidate drugs.
SIM0417 is a small-molecule antiviral agent that targets the 3C-like protease, a key enzyme in the replication and life cycle of coronaviruses, including SARS-CoV-2. Preclinical animal models have demonstrated good antiviral activity and the safety of SIM0417. Due to the 3CL protease’s highly conserved nature, SIM0417 has a strong inhibitory effect on a variety of SARS-CoV-2 variants including the highly infectious Delta variant.
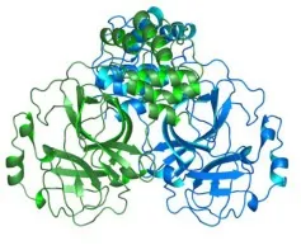
As it’s a common and fundamental structure of coronaviruses, 3CL’s highly conserved nature is also very unlikely to change as the virus mutates, implying the effectiveness of SIM0417 may be similarly applicable to future variants as well.
Mr. Ren Jinsheng, Chairman and CEO of Simcere commented, “R&D collaboration is one of our ‘dual-track’-based R&D strategies. SIMM’s powerful platforms and technologies for drug development have produced several novel drug candidates for COVID-19 during the pandemic. Simcere is committed to providing today’s patients with medicines of the future. We hope this collaboration will accelerate the transformation of research results coming from the lab into clinical studies. We are very excited to work with CAS to develop this new generation of antiviral drugs and help further contribute China’s strength to fight against COVID-19.”
About Simcere Pharmaceutical Group
Simcere Pharmaceutical Group limited (2096.HK) is rapidly transitioning to an innovation and R&D-driven pharmaceutical company, with the mission of “providing today’s patients with the medicines of the future.” With an established a national key laboratory of translational medicine and innovative pharmaceuticals, Simcere focuses on the three main therapeutic areas of oncology, central nervous system diseases and autoimmune diseases. The company has developed a diversified product portfolio and industry-leading capabilities through its vigorous in-house R&D efforts and extensive R&D collaborations. In an effort to bring more global life science breakthroughs to China, Sincere continues strategically collaborate with world leading pharmaceutical companies and biotechnology companies.
For more information, please visit www.simcere.com.
About Shanghai Institute of Materia Medica, Chinese Academy of Sciences
The Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences (CAS) was founded in 1932, and has the longest history as a comprehensive research institution for drug discovery in China. SIMM focuses on the frontiers of life sciences while aiming to help solve key scientific problems in drug discovery. As such, SIMM carries out both basic and applied studies to develop new theories, methods and technologies. In recent years, through the establishment of a comprehensive, advanced and efficient R&D system, SIMM has promoted a series of innovative drugs for the treatment of cancer into clinical trials, including those for cardio-cerebrovascular disease, neuropsychiatric disease, metabolic disease, autoimmune disease, and infectious disease.
For more information, please visit http://www.simm.ac.cn/.
Forward Looking Statements
Information set forth in this press release contains forward-looking statements, which involve a number of known and unknown risks, uncertainties and assumptions. The forward-looking statements contained herein reflect the current judgment and views of Simcere Pharmaceutical Group Limited as of the date of this press release. Such forward-looking statements are neither promises nor guarantees but are subject to a variety of risks and uncertainties, many of which are beyond our control, or may not materialize, and which could cause actual results to differ materially from those contemplated in these forward-looking statements. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any such statements, whether as a result of new information, future events or otherwise, to reflect any change in our expectations or any change in events, conditions or circumstances on which any such statement is based.