On April 15, 2022, Simcere Pharmaceutical(2096.HK), an innovation and R&D driven pharmaceutical company in China held its first R&D day for international investors, during which the executive leaders revealed Simere’s R&D strategy,and gave an update on Simcere’s pipeline to the global investment community.
Mr. Jinsheng Ren, Chairman and CEO,Dr. Renhong Tang, Executive Vice President,Dr. Bijoyesh Mookerjee, Chief Medical Officer of Oncology,Mr. Gaobo Zhou, Chief Investment Officer,Dr. Kevin Oliver, Senior Vice President,Dr. Danny Chen,Senior Vice President, Mr. Andrew Zhu,Senior Vice President,and Mr. Jun Bao,Board Secretary attended the meeting as speakers.

Highlights:
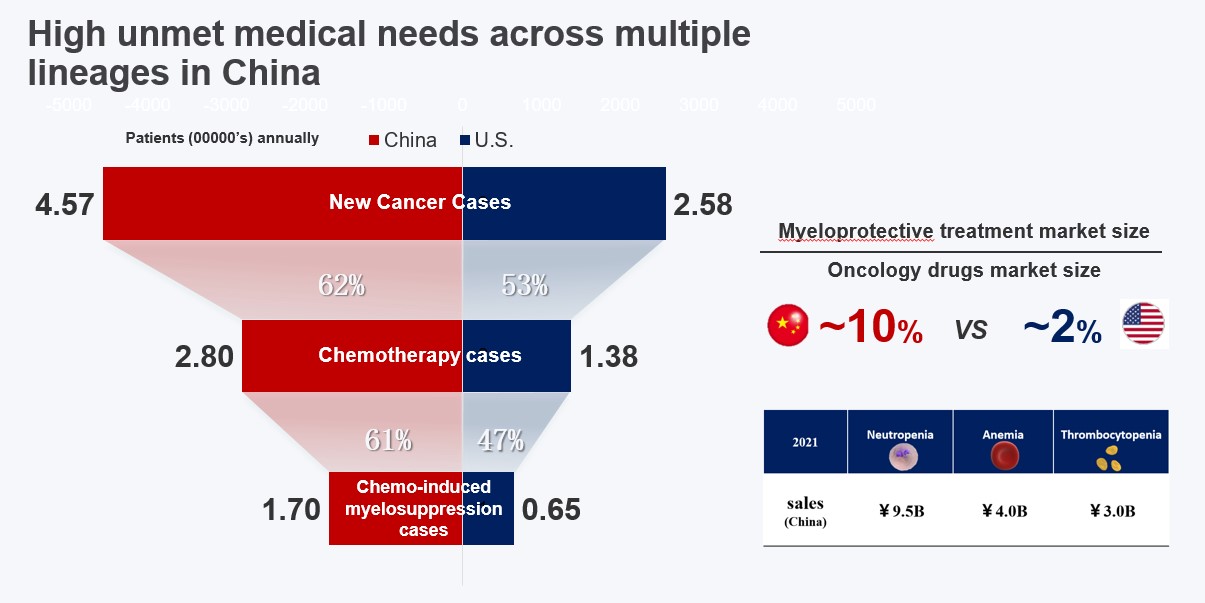
First-in-class (FIC) novel myelo-protective drug Trilaciclib is expected to be launched in China in 2022. Targeting large Chinese patient population with certain common cancers who need to be treated with chemotherapy, the product has the potential of providing substantial clinical benefit and gaining a huge market share.
As a breakthrough product in China’s stroke market,Sanbexin® achieved significant growth and contributed to the overall annual CNS business revenue of RMB 1.5 billion, highlighting Simcere’s strong commercial capabilities. Rapid clinical development of Sanbexin sublingual tablets for sequential stroke treatment and other investigational new drug are forming a multi-mechanism treatment approach to stroke which will further secure Simcere’s leadership in this therapeutic area.
Proprietary oral SARS-CoV-2-3CL inhibitor co-developed by Simcere and the Shanghai Institute of Materia Medica(SIMM)of the Chinese Academy of Sciences is the first locally manufactured oral 3CL inhibitor approved for clinical trial in China. The first subject was enrolled in early April.
Simcere’s robust R&D pipeline consists of nearly 60 projects, with 20 projects at clinical stage, involving 17 potential innovative drug products.
The company is seeking global expansion via diversified strategies including expanded BD&L, planning for global multiple center clinical trials, building a global executive team and recruiting key and diverse talent worldwide.
01 Successful transformation towards innovation that emphasizes on “effectiveness"
On the journey to build an innovation and R&D-driven pharmaceutical company, Simcere emphasizes its focus on "clinical differentiation and effectiveness”. Simcere has marketed five innovative drugs in China and in 2021, the share of innovative products in total revenue reached 62.4%. Annual R&D investment was RMB 1.417 billion, accounting for 28.3% of total revenue.
Currently the company has an R&D pipeline of nearly 60 innovative projects. The two-pronged approach of Simcere’s R&D, combining independent research with collaborative development, focuses on oncology, CNS and autoimmune diseases, with an eye on additional disease areas that may have substantial future clinical needs.
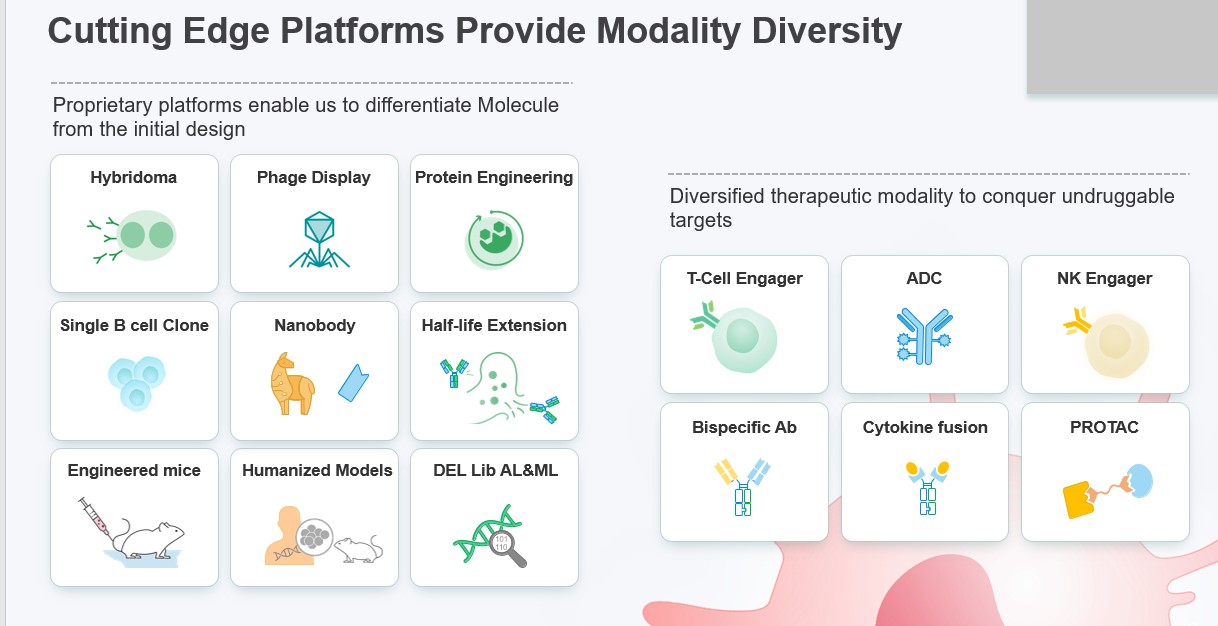
Simcere's independent R&D technology platform has deployed multiple cutting-edge technologies including immuno-oncology bispecific antibodies, multi-specific T cell engagers, synthetic lethality molecules, drug targets identified through artificial intelligence (AI), Treg-biased fusion proteins, and autoimmune antibody-drug conjugates (ADCs) etc. 6 projects independently developed by Simcere were presented at the 2022 AACR annual meeting, including two oral presentations. 10 new INDs are expected to be filed in China or in the United States, more than half of which are driven by in-house development.
In response to the major challenges of human health posed by COVID-19, Simcere is co-developing an oral anti-SARS-CoV-2 virus agent SIM0417 in collaboration with SIMM at the Chinese Academy of Sciences. It is the first proprietary anti-COVID 19 oral 3CL inhibitor developed locally in China that has been approved for clinical trials. The phase I clinical study has started subject enrollment in Qianfoshan Hospital in Shandong Province. The Phase I clinical study is expected to complete dosing of all subjects by the end of May 2022.
SIM0417 is highly selective and potent small molecule inhibitor of 3CL, a key enzyme in the replication and life cycle of coronaviruses, including SARS-CoV-2. Preclinical studies have demonstrated good antiviral activity and safety. Due to the 3CL protease’s highly conserved nature, SIM0417 has a strong inhibitory effect on a variety of SARS-CoV-2 variants including wild type virus, the Delta variant, and the Omicron variant.
In face of the Omicron infection which appears far more contagious, though less severe clinically, than the infection caused by wild type or Delta strains, Simcere is designing creative phase 2/3 clinical studies for wider target populations using a differentiated strategy potentially compared with standard of care or investigational drugs on the similar target.
02 Oncology: Trilaciclib rapidly progressing towards market approval at “Simcere speed”
Simcere’s oncology R&D strategy is to focus on highest unmet clinical needs, explore opportunities of first and best in class medicines, and deliver new assets and combinations. The company’s oncology pipeline covers indications for several malignancies including but not limited to lung cancer, breast cancer, gastrointestinal tumors, gynecological malignancies, and gliomas, with novel MOAs and differentiated development strategies, such as with an IO checkpoint inhibitor that can be administered subcutaneously (Simcere’s partnered Envafolimab), Short-acting CDK4/6 cell cycle inhibitor (Trilaciclib co-developed with G1 therapeutics), monoclonal antibody acting on IO novel target TNFR2 (SIM0235), and blood-brain barrier permeable potential best-in-class(BIC) oral SERD compound (SIM0270).
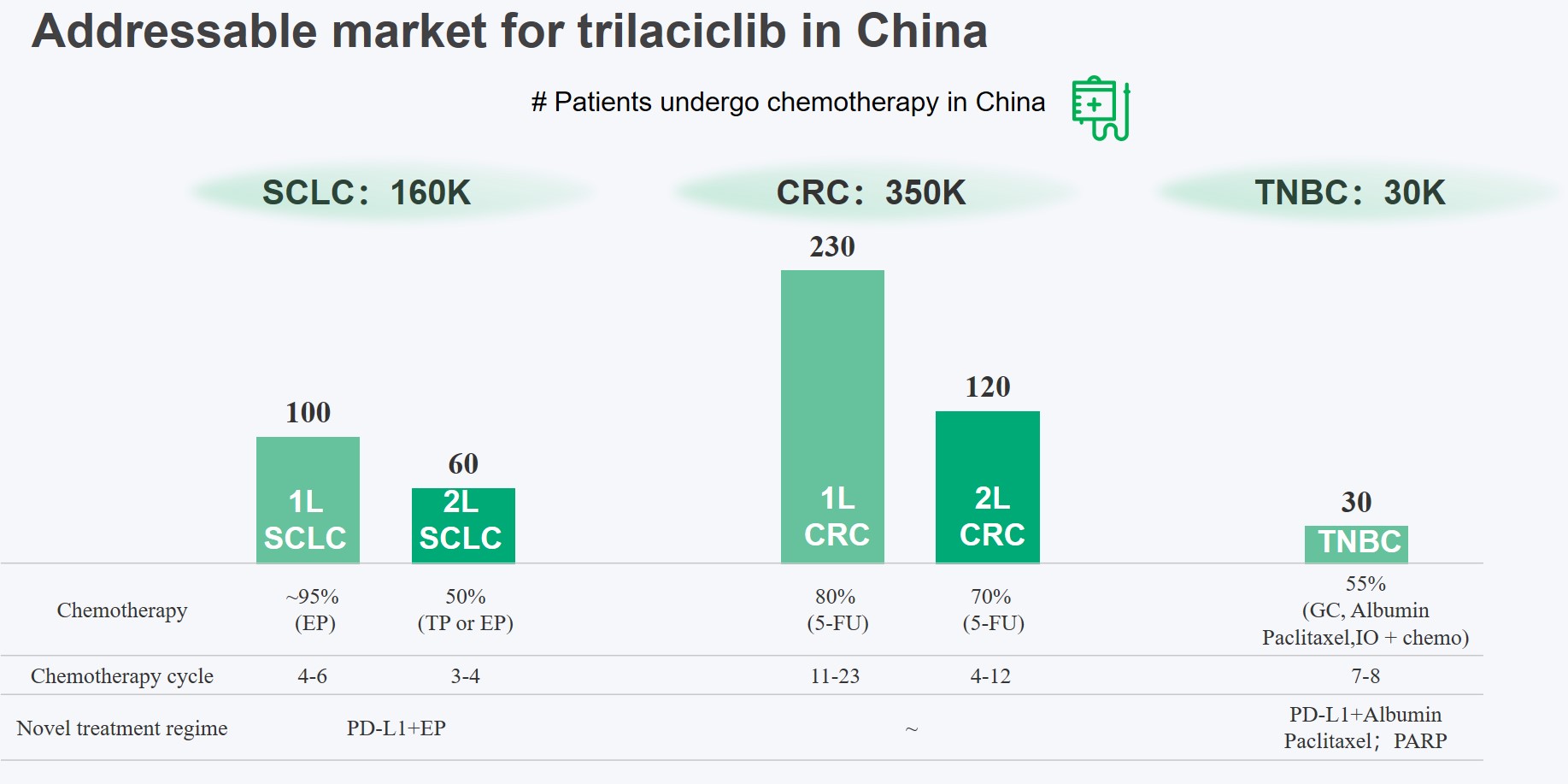
Trilaciclib (COSELATM) jointly developed by Simcere and G1 Therapeutics has initiated phase 3 clinical trials in China for the treatment of extensive-stage small cell lung cancer (ES-SCLC), colorectal cancer, and triple-negative breast cancer. The registration study of ES-SCLC has met its primary end point, which further validates the safety and myelo-protective efficacy of trilaciclib, on granulocytes, platelets and red blood cell lines in the Chinese population.
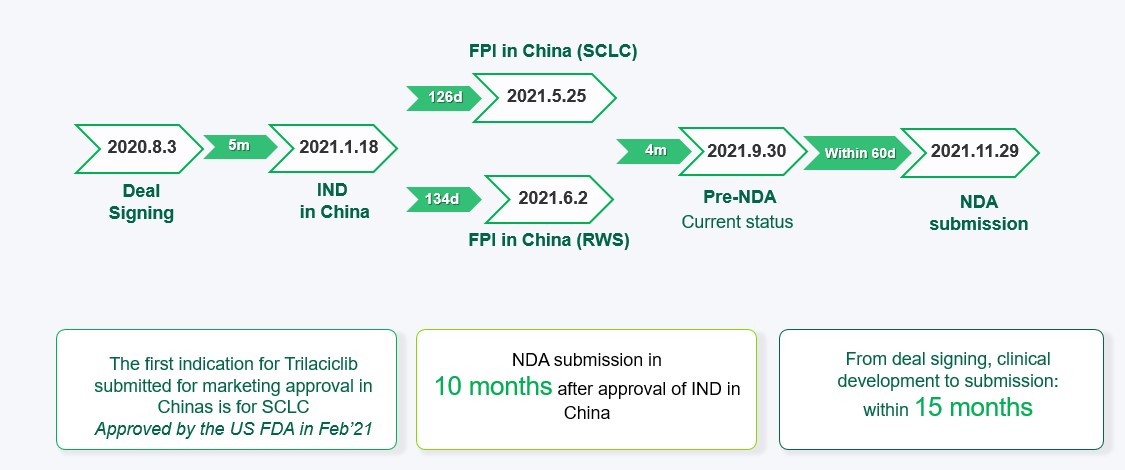
The NDA for the first indication submitted in China (small cell lung cancer) has been included in priority review by the CDE. It is expected to be approved in the latter half of 2022. Simcere was able to submit NDA submission in China within 10 months of the IND approval thereby demonstrating a highly efficient and expeditious conduct of clinical development. Targeting large Chinese patient population receiving chemotherapy, the product has the potential for delivering significant patient impact and commercial success.
The submission was additionally supported by Real Word Study evidence obtained in Hainan where Chinese patients can receive drugs approved by the FDA or EMA prior to approval in China. 30 patients were enrolled from June 2021 to November 2021 and data analysis will be completed in 2022.
03 CNS:Multi-mechanism approach to secure leadership in stroke, with Sanbexin® as core product
Stroke, a disease causing 3.3 million new cases in China annually, is a major CNS illness with a highly unmet clinical need. Simcere has been deeply involved in the field of stroke treatment for decades. Sanbexin® independently developed by Simcere is the only new stroke drug approved for market in the world since 2015. The product achieved market excellence and has helped over 600,000 Chinese patients within its first year of launch.
Positive results from the pivotal phase 3 study (TASTE study) of Sanbexin® for acute ischemic stroke (AIS) were published in the journal STROKE in 2021. Subject enrollment in the phase 4 study (TASTE II study) of Sanbexin® combined with vascular recanalization therapy was initiated in March 2022. Clinical exploration of Sanbexin® in hemorrhagic stroke is expected to be launched in June 2022.
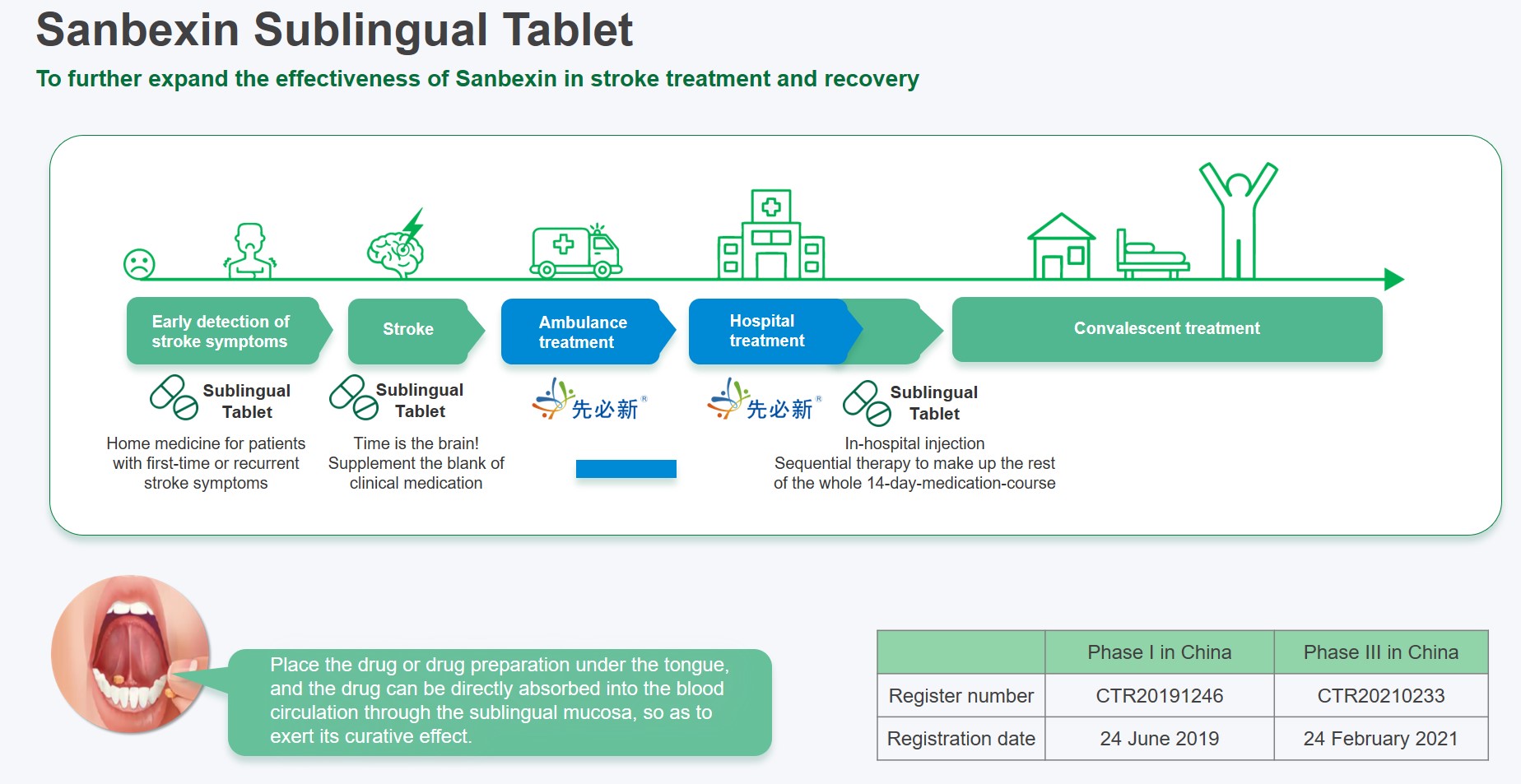
Phase III registration study of Sanbexin sublingual tablet has nearly completed enrollment, with topline clinical data expected within 2022. The sequential treatment of Sanbexin® oral plus injection dosage form will likely cover the entire course of ischemic/hemorrhagic stroke, and enable patients to receive timely and complete treatment.
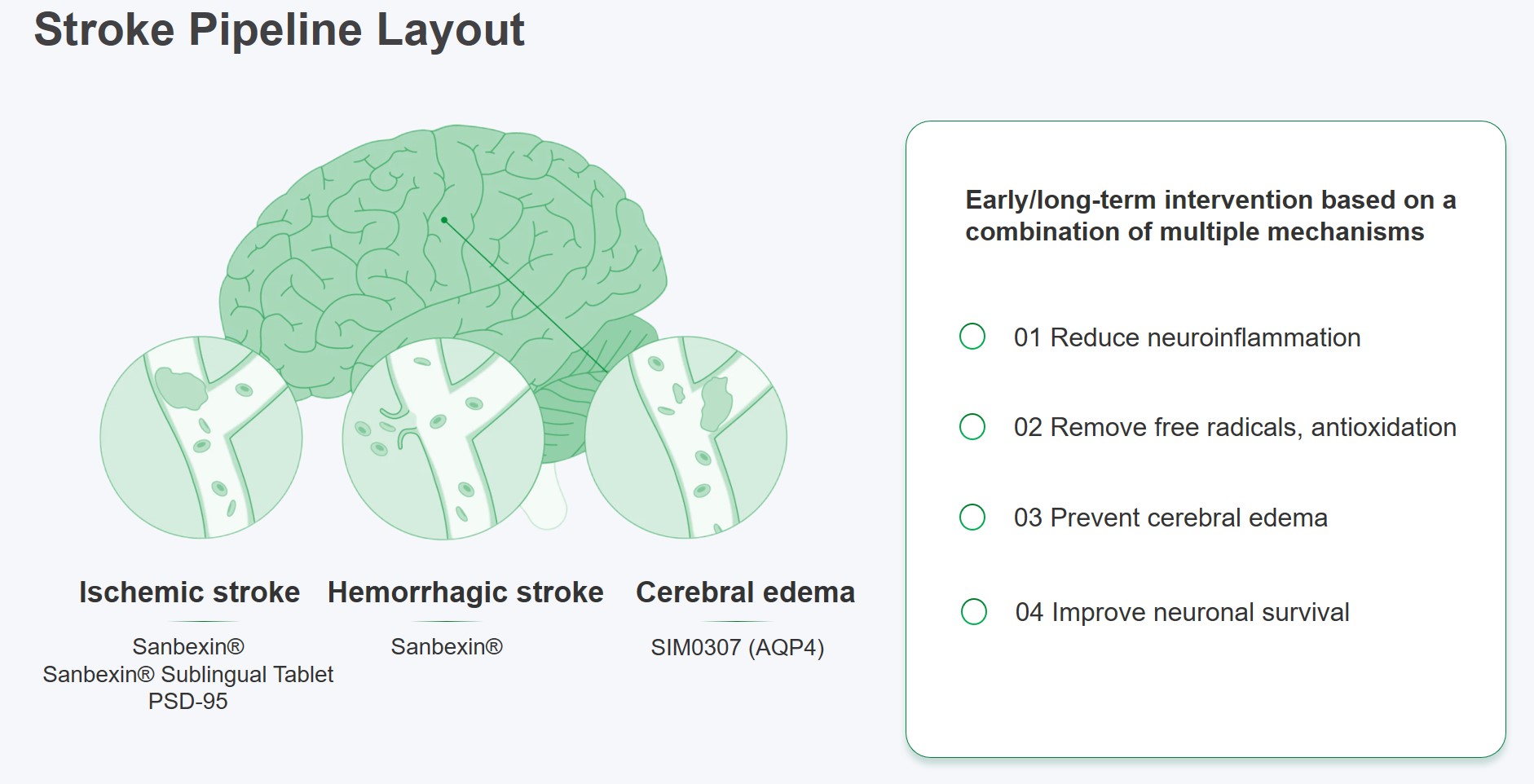
Furthermore, Simcere will secure its leadership in stroke in China by forming a combination of multiple mechanism assets, including SIM0307, an AQP 4 inhibitor for brain edema that entered phase 1 clinical trials in China;and the regional licensing of AVLX-144, an innovative drug for acute ischemic stroke targeting the postsynaptic scaffold protein(PSD)-95.
In addition, Simcere entered Alzheimer's disease (AD) field through partnering two programs with Vivoryon. The oral small molecule SIM0408 can inhibit the production of neurotoxic N3pE amyloid, which plays a role in the pathology of early stages of AD. It has received IND approval in China and may bring more effective medication to millions of Chinese patients who suffer from this currently cureless disease.
04 Go global, reach new heights
The preliminary success of Simcere’s transformation towards innovation is followed by further globalization efforts that will be accelerated by global business development and licensing, astute investments, global R&D expansion, and through recruiting key international talent.
The BD&L team of Simcere is geographically established in China, the United States, the United Kingdom, Germany, etc., and processes high credibility, enabled by diversified executive background experiences and deep reputation in the industry.

Aiming at becoming ‘Partner of Choice’, Simcere values its existing 35+ alliances and LP positions globally. Since 2021, more than 8 licensing partnerships were executed to introduce innovative assets into the pipeline. Simcere also strategically participated in a number of international life science investment funds, and is thereby closely associated with some of the world's most active innovation communities within biotech and biopharma.
Multiple in-licensed products have achieved impressively rapid progress in clinical study in China, including trilaciclib, a short-acting CDK4/6 inhibitor, SIM0307 a molecule for cerebral edema, and SIM0408 a novel Alzheimer’s disease therapy. Simcere’s most recently announced licensing agreement with Lynk pertaining to a highly selective JAK 1 inhibitor which is expected to bring another high potential product for autoimmune diseases.
In terms of R&D internationalization, Simcere has established 4 innovation centers in China and the United States to explore the global development of novel medicine SIM0235, the TNFR2 antibody developed by Simcere has received IND approval in both China and the United States. The anti-COVID 19 oral 3CL inhibitor is also expected to start clinical trials overseas soon. In 2022, 3 new INDs of internally developed assets are expected to be submitted in the United States.
Simcere is also building a team of leading talents with global focus and visions. Among the speakers at the R&D Day, Dr. Renhong Tang, Dr. Renhong Tang, Executive Vice President, Dr. Kevin Oliver, SVP and Head of Global BD&L, Mr. Zhou Gaobo, Chief Investment Officer, Dr. Bijoyesh Mookerjee, Chief Medical Officer of Oncology, Dr. Danny Chen, Senior Vice President and Mr. Andrew Zhu, Senior Vice President who recently joined Simcere all hold decades of academic and professional experience in large multinational companies. Their expertise will help Simcere's innovation and globalization strategy as well as the mission to bring future medicines to today’s patients.

At the Q&A session hosted by Board Secretary Mr. Jun Bao, Simcere’s executive team members answered questions raised by investors.