Simcere Pharmaceutical Group, a pioneer in innovative healthcare solutions, proudly announces a significant achievement for its Class 1 innovative drug, Sanbexin® (edaravone and dexborneol concentrated solution for injection). The esteemed "Chinese Guideline of Clinical Management for Cerebral Vascular Diseases" (2nd version), commonly referred to as the "New Guideline," has bestowed a Class IIa recommendation upon Sanbexin®, marking a pivotal recognition in the realm of stroke treatment. This distinguished acknowledgment was officially unveiled during the 9th Annual Scientific Session of the Chinese Stroke Association & Tiantan International Stroke Conference 2023 (CSA&TISC 2023), a prominent event held at the China National Convention Center in Beijing on June 24.
The "New Guideline" introduces a groundbreaking paradigm shift in the approach to acute ischemic stroke (AIS) treatment by replacing the conventional concept of "neuroprotection" with the advanced notion of "brain cell protection." Distinguished experts from the Chinese Stroke Association (CSA) editorial team, who helmed the creation of the "New Guideline," emphasized Sanbexin®'s role in this new perspective. Drawing from robust evidence, they awarded Sanbexin® a Class IIa recommendation due to its unparalleled potential in enhancing the clinical outcomes of AIS patients.

The "Chinese Guideline of Clinical Management for Cerebral Vascular Diseases" (2nd version) was released on June 24
Professor Xu Anding from the First Affiliated Hospital of Jinan University, the driving force behind the "New Guideline," emphasized the imperative to accelerate the integration of high-quality clinical research achievements into diagnostic and treatment practices. This concerted effort aims to bridge regional and structural disparities in medical technology while fostering standardized clinical practices. By meticulously screening, demonstrating, and analyzing groundbreaking research in the neurological field, the expert committee comprehensively overhauled and upgraded the 2019 version of the guideline, a pivotal step toward elevating the overall quality of neurological care in China.
The journey to this milestone began in June 2019 with the release of the "Chinese Guideline of Clinical Management for Cerebral Vascular Diseases" (1st version). Fueled by the constant emergence of new clinical evidence in cerebrovascular disorders, including seminal studies involving the Chinese population, the Standing Council of CSA initiated the collaborative effort to craft the "New Guideline" in early 2022. This initiative aimed to harness the latest clinical evidence to enhance the prognosis of patients through optimized clinical management of cerebrovascular disorders.
Professor Dong Qiang, an esteemed figure from Huashan Hospital, affiliated with Fudan University, highlighted the limitations of the traditional "neuroprotection" concept. In contrast, the "New Guideline" champions the safeguarding of neurovascular units, encompassing neurons, glial cells, endothelial cells, and pericytes. This holistic approach, rooted in contemporary treatment ideologies, aligns seamlessly with the inclusion of high-quality research findings within China. The recommendation for Sanbexin®, which hinges on robust medical evidence, resonates with this updated treatment paradigm.
Sanbexin® (edaravone and dexborneol) stands as a beacon of innovation in stroke treatment, securing its position as the sole Class 1 innovative drug to receive marketing approval in the stroke treatment domain since 2015. Anchored in a multifaceted mechanism of action, including free radical scavenging, anti-inflammatory prowess, and blood-brain barrier protection, Sanbexin® uniquely curtails brain cell damage and dysfunction engendered by ischemia and hypoxia in stroke patients. This extraordinary therapeutic option ushers in new avenues for improved patient outcomes.
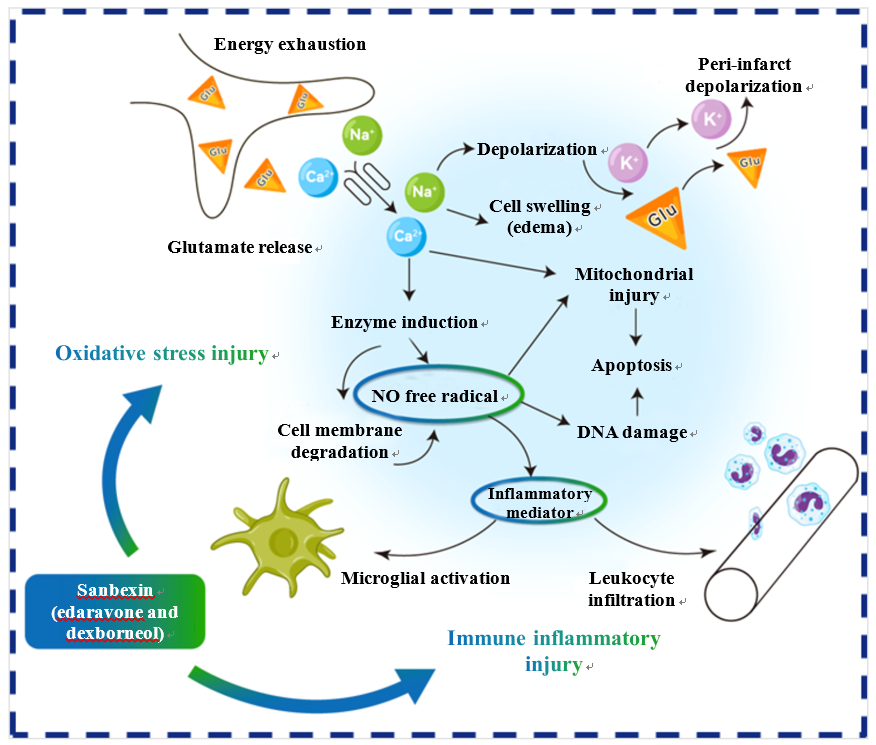
Diagram of the mechanism of action of edaravone and dexborneol
Stroke remains an imposing global health challenge, ranking as the second-largest cause of mortality worldwide and an acute concern within China. The profound impact of stroke, characterized by a high recurrence rate, disability prevalence, and mortality, underscores its societal toll. Notably, Sanbexin® has transformed the landscape of stroke treatment, benefiting millions of patients in the three years since its introduction.
Simcere Pharmaceutical Group's unwavering commitment to groundbreaking medical solutions, exemplified by Sanbexin®, continues to reshape the landscape of stroke treatment, offering hope and relief to countless individuals grappling with the complexities of cerebrovascular diseases.