March 12, 2024. Simcere Zaiming, an innovative oncology company and a subsidiary of Simcere Pharmaceutical Group Ltd., announced that it has received approval both from the U.S. Food and Drug Administration (FDA) and the China National Medical Products Administration (NMPA) for the investigational new drug candidate SIM0500, to carry out clinical trials in patients with relapsed or refractory multiple myeloma (MM).
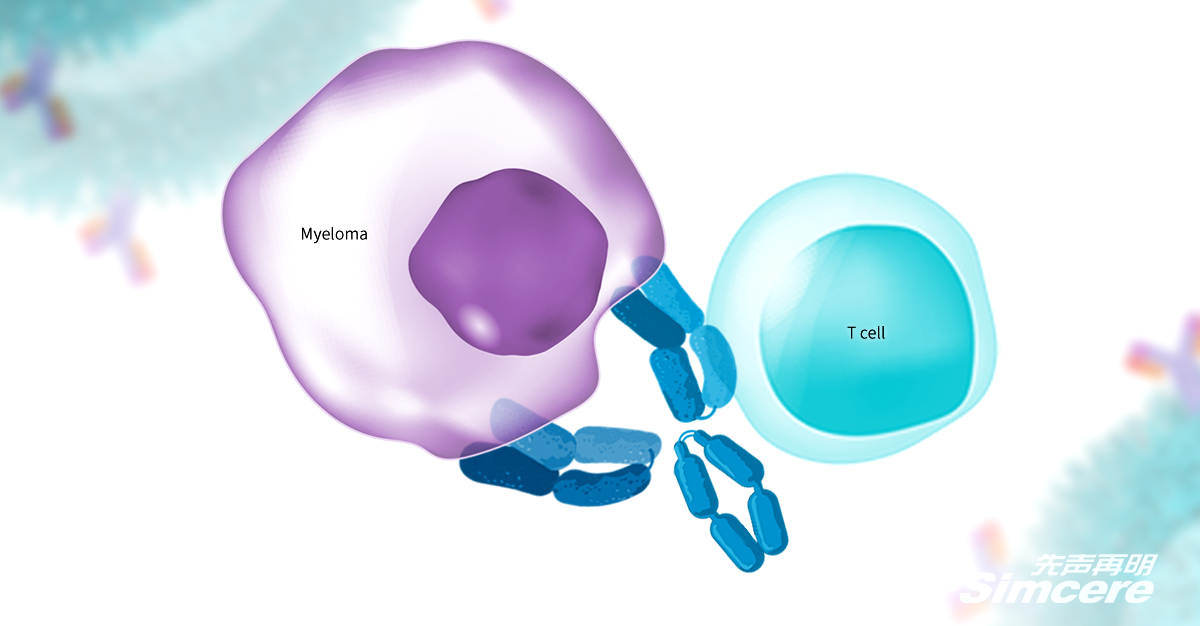
SIM0500 is a humanized GPRC5D-BCMA-CD3 tri-specific antibody independently developed through Simcere Zaiming’s T-cell engager polyspecific antibody technology platform. By combining a low affinity/high target activating CD3 antibody, along with 2 tumor-related antibodies(GPRC5D, BCMA), this novel T cell-activating molecule specifically targets MM tumor cells and has an enhanced tumor suppression activity through the synergy of multiple target effects.
Data from preclinical studies has demonstrated a favorable tumor-killing effect and good tolerance of SIM0500 even at low doses. In animal models, no tumor recurrence is observed after drug discontinuation. It is potentially a best-in-class candidate that may provide new therapeutic choices to overcome the drug resistance caused by existing treatments of MM.
About Simcere Zaiming
Simcere Zaiming is an oncology-focused biopharmaceutical company and a subsidiary of Simcere Pharmaceutical Group Limited (HKEX: 2096, “Simcere”). Founded in 2023, Simcere is committed to developing disruptive therapies that have the potential to address unmet clinical needs for cancer patients around the world. Simcere Zaiming has built an innovative R&D pipeline, with differentiated clinical assets. Several products of Simcere Zaiming are commercially available in China, which include COSELA®, Endostar®, and Envafolimab. Simcere Zaiming strives to bring potentially breakthrough treatment options to cancer patients worldwide through in-house R&D, manufacturing, and commercialization, as well as collaborations with global partners of innovative cancer therapeutics.
Media contact: pr@zaiming.com