Sanbexin Sublingual tablets is a combination of Edaravone and Dexborneol therapy which has received US FDA breakthrough therapy designation for AIS
On November 2, 2024, SimcOn November 2, 2024, Simcere Pharmaceutical announced that Sanbexin® sublingual tablets (generic name: edaravone and dexborneol sublingual tablets), an innovative drug for stroke, has been approved for marketing by the National Medical Products Administration. This product is indicated for the improvement of neurological symptoms, daily activities, and functional impairment due to acute ischemic stroke.
Sanbexin® sublingual tablets is a dual-target brain cytoprotective agent composed of edaravone and dexborneol. These two active ingredients exert synergistic anti-oxidant and anti-inflammatory effects, which can significantly reduce brain cell damage caused by acute ischemic stroke.
The sublingual tablets are designed for quick disintegration once in contact with saliva under the tongue. This facilitates the active ingredients’ rapid absorption into the blood and brain through the sublingual venous plexus. Compared to conventional oral formulations, sublingual tablets bypass the first-pass hepatic metabolism, which is conducive to higher drug bioavailability and faster onset of action.

Packaging of Sanbexin® sublingual tablets
Previously, Sanbexin® injection was approved for marketing in China in 2020. As the world's only innovative drug approved for stroke since 2015, it has helped more than 3 million patients in the past 4 years.
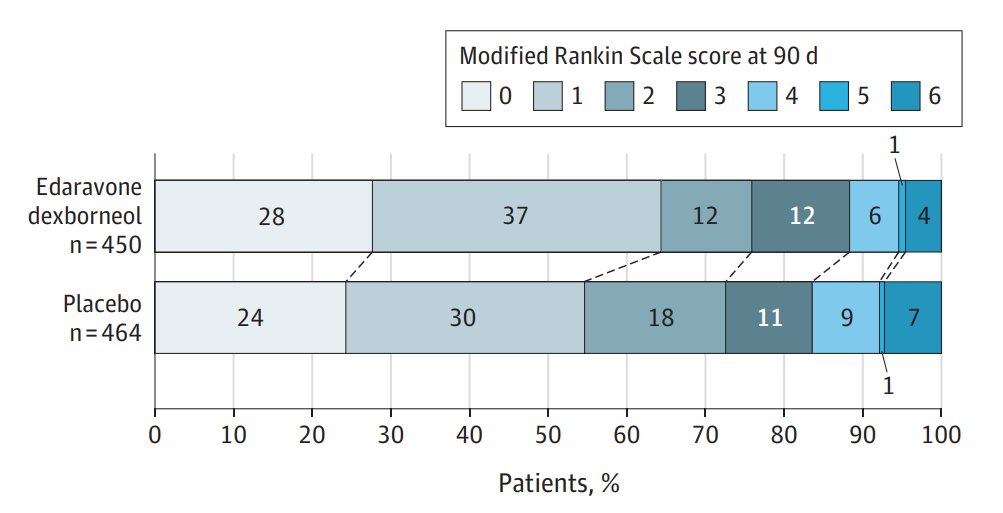
the primary endpoints of the TASTE-SL study
The phase III clinical trial led by Professor Fan Dongsheng of Peking University Third Hospital showed that the patients in the Sanbexin® sublingual tablets group after 14 consecutive days of drug administration, obtained a significantly higher proportion of functional independence outcome at 90 days post-treatment than those in the placebo group (64.4% vs. 54.7%,). The latest data was published in JAMA Neurology on February 19, 2024.

Professor Fan Dongsheng, Principal Investigator of TASTE-SL and Professor at Peking University Third Hospital mentioned:"Sanbexin® sublingual tablets has shown significant effects and good safety in improving recovery of cerebral cells and independent living ability during the acute phase in patients with acute ischemic stroke. The more convenient administration allows for sequential therapy with Sanbexin® injection, facilitating stroke patients to receive a complete course of brain cytoprotection in and outside of the hospital during the acute phase of stroke."

Professor Wang Yongjun, Director of Beijing Tiantan Hospital, Capital Medical University commented:"The average length of hospital stay for stroke patients in China is about one week, while clinical studies suggest that brain cytoprotective drugs need to be used for 14 consecutive days. Sanbexin® sublingual tablets is easy to take, allowing patients to receive treatment at home. This can better reduce disability among patients and is also expected to lower medical costs."
In August 2024, Sanbexin® sublingual tablets was granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration (FDA) for AIS, making it the world's first innovative drug in the field of stroke treatment to have received such acknowledgment. Currently, a global multi-centered clinical trial of Sanbexin® sublingual tablets is under preparation.

Sanbexin® sublingual tablets receive FDA Breakthrough Therapy Designation for AIS

Dr. Marc Fisher, former President of the World Stroke Organization and Professor at Harvard Medical School,commented on this new approval:"Sanbexin® has gradually gained popularity in China and is now available in a sublingual tablet formulation, with clinical data confirming its safety and efficacy. We are hoping to see trials of Sanbexin® sublingual tablets conducted outside China. If the trial results are positive and it receives approval in other countries, such as the U.S., it will have a huge impact globally on the treatment of acute ischemic stroke."

"The approval of Sanbexin® sublingual tablets in China is believed to significantly reduce the number of stroke-related disabilities in China."Professor Gregory W. Albers, Director of the Stroke Center and the Medical Center at Stanford University commented, " We are planning to conduct a large-scale Phase III clinical trial of Sanbexin® sublingual tablets in the United States, hoping to replicate the success of the trial in China and help reduce the global burden of stroke-related disabilities."
The Sanbexin® sublingual tablets, with its convenient delivery method, will make stroke prevention and treatment more comprehensive and accessible. Its therapeutic area is expected to be expanded to pre-hospital emergency treatment for the acute phase of stroke, as well as to the treatment for the sub-acute and chronic phases of cerebrovascular diseases, to further promote the recovery of neurological functions and to improve the functional prognosis of stroke patients.