On Aug. 23, 2023, Simcere Pharmaceutical Co., Ltd. (“Simcere”) and Lynk Pharmaceuticals (Hangzhou) Co., Ltd. ( “Lynk Pharmaceuticals”) announced positive topline data a Phase II clinical trial of LNK01001 for the treatment of ankylosing spondylitis (“AS”). LNK01001 was jointly developed by Simcere and Lynk Pharmaceuticals. AS is a chronic inflammatory disease, typically afflicting patients in their twenties and thirties and seriously affecting their mobility and quality of life. There are around five million AS patients in China and the clinical needs are largely unmet.
On March 18, 2022, Simcere and Lynk Pharmaceuticals announced a strategic commercialization partnership to develop and commercialize LNK01001. Under the terms of the agreement, Lynk Pharmaceuticals is responsible for the development of LNK01001 and Simcere obtains the exclusive rights to market LNK01001 for rheumatoid arthritis (“RA”) and AS in China.
On May 16, 2023, LNK01001 achieved positive results from a Phase II clinical trial for the treatment of RA, demonstrating statistically significant differences in efficacies compared to placebo in both primary and key secondary endpoints and good safety and tolerability.
LNK01001 the selective JAK1 inhibitor. JAK1 belongs to an important subgroup of the Janus kinase (JAKs) family that is closely related to the pathogenesis of various autoimmune diseases. Multiple JAK inhibitors have been on the market for autoimmune diseases and, according to Frost & Sullivan, the JAK inhibitor market will reach $30.5 billion by 2030. The preclinical data show that LNK01001 has a higher selectivity and potentially better safety than other marketed JAK inhibitors. It is expected to provide RA, AS, and other patients with a newer, safer, and more effective treatment.
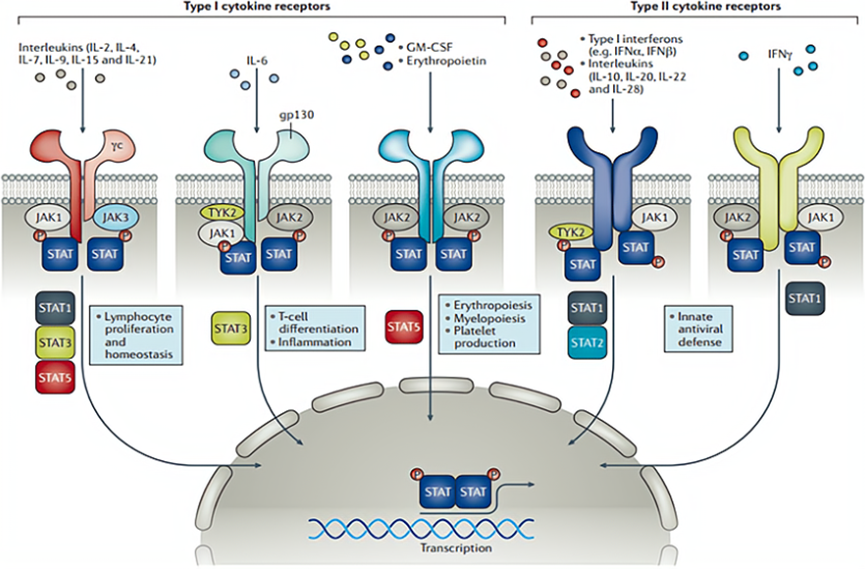
JAKs Acting Mechanism
This study with positive topline data was a multi-center, randomized, double-blind, placebo-controlled Phase II clinical study conducted in adult AS patients and led by Professor Zeng Xiaofeng, the director of the Department of Rheumatology and Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences. A total of 177 AS patients with inadequate response to or intolerance to NSAID were enrolled. The primary efficacy endpoint is the ASAS40 response* rate after 12 weeks of treatment.
The AS trial showed that the patient conditions in both the low dose and high dose groups with LNK01001 improved markedly, with efficacies exceeding all projected primary and critical secondary endpoints after 12 weeks of treatment and demonstrating statistically significant differences compared to placebo. In addition, LNK01001 is proved to be fast-acting, with both high and lose dose groups showing varying degrees of improvement in the ASAS40 response rate of various efficacy endpoints from week 2. Moreover, the overall safety and tolerability of LNK01001 were very good. The majority of treatment-emergent adverse events (TEAEs) were mild, with no serious adverse advents (SAEs) reported in the LNK01001 groups.
According to Professor Zeng Xiaofeng, the lead principal investigator, “AS is a chronic inflammatory disease that mainly affects the sacroiliac joint at the spine and pelvis. The primary therapeutic objective is to control or reduce inflammation and relieve pain and stiffness. The good efficacy and safety shown in this study will be further confirmed in a Phase III clinical study to benefit more patients.”
Strategically focusing on autoimmune diseases, Simcere is excited about the significant therapeutic effects of LNK01001 in the treatment of AS patients and looks forward to building a powerful alliance with Lynk Pharmaceuticals, quickly advancing LNK01001 towards commercialization, and affording all AS patients in China with a safer and more effective treatment.
[*] ASAS40 response: A ≥ 40% improvement from baseline and an absolute improvement of ≥ 2 units (range 0–10) in ≥ three of the following four domains: patient global assessment of disease activity, back pain, physical function, and inflammation, without any worsening in the remaining domain.
About Lynk Pharmaceuticals
Lynk Pharmaceuticals, a clinical-stage company, was founded in 2018 by senior drug R&D experts and executives from Pfizer, Merck and Johnson & Johnson. Lynk Pharmaceuticals is dedicated to the discovery and development of innovative drugs for the treatment of cancer, as well as immune and inflammatory diseases. Driven by a higher goal, Lynk Pharmaceuticals aims to be a market leader in addressing unmet medical demands through the development of innovative therapies. To date, Lynk Pharmaceuticals has independently developed a number of Class I clinical innovative new drugs and launched a number of clinical studies globally.
For more, visit www.lynkpharma.com