On Aug. 26, 2023, SIM0278, an innovative interleukin 2 mutant fusion protein (IL-2 mu-Fc) independently developed by Simcere Pharmaceutical (2096. HK), completed its first subject dosing at the Affiliated Hospital of Qingdao University.
Led by Director Cao Yu of the Affiliated Hospital of Qingdao University, this randomized, double-blind, sponsor-open, placebo-controlled, dose-escalation Phase I clinical study aimed at evaluating the safety, tolerability, and pharmacokinetic and pharmacodynamic properties of SIM0278 in healthy Chinese subjects.
On July 27, SIM0278 was approved by the National Medical Products Administration (NMPA) for clinical trials indicated for moderate-to-severe atopic dermatitis (AD). After active preparation, Simcere Pharmaceutical reached the milestone of the first subject dosing within one month, leading the industry in the speed of enrollment. If clinically validated, SIM0278 may make up for the lack of approved AD therapies and meet clinical needs for continuous medication.
AD is a chronic relapsing inflammatory skin disease, with typical clinical symptoms such as dry skin, erythema, edema, erosion, exfoliation, and lichenification[1]. According to Frost & Sullivan, the global AD patient population reached 649 million in 2019 and is expected to increase to 755 million in 2030. A variety of therapeutic methods are available to alleviate or eliminate the clinical symptoms of AD and to reduce and prevent relapses. Still, topical medications have limited efficacy and immunosuppressants and biologics are associated with multiple adverse reactions or risks[2-5]. A safer and more effective medication is still necessary to meet clinical needs for disease control and long-term treatment.
Studies have shown that regulatory T cells (Tregs) are involved in the pathogenesis of AD. Tregs play an essential role in immune tolerance and immune regulation. It is critical for the down-regulation of inflammation in skin anaphylaxis and the maintenance of peripheral immune tolerance. InterleukinI-2 (IL-2) involves the differentiation, expansion, and survival of Tregs[6].
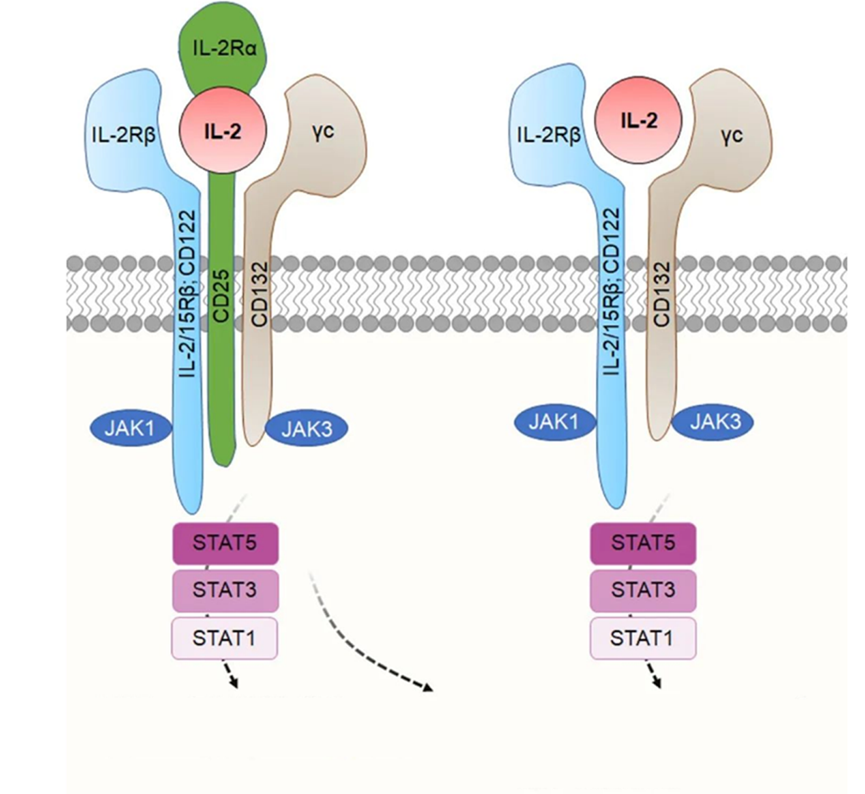
Signaling pathway of IL-2 (Drawn by Fu Yong, Department of In Vitro Pharmacology, Simcere Pharmaceutical)
SIM0278, a Treg-preferring IL-2 mu-Fc developed at the internal protein engineering platform of Simcere Pharmaceutical, reduces the affinity to effector T cells but retains the high affinity to Tregs through the introduction of the corresponding mutations, which in turn improves the selectivity for Tregs.
Preclinical studies have shown that SIM0278 can selectively activate Tregs in vitro, with no effect on effector T cells or natural killer cells, thus restoring the immune balance of the body.
Moreover, in addition to the approved AD indication, SIM0278 is potentially indicated for a variety of autoimmune diseases and is likely to benefit more than ten million patients in China. Preclinical trials have also shown that SIM0278 promises to be more effective, longer-lasting, and safer.
In Sep. 2022, Simcere Pharmaceutical granted Almirall an exclusive right to develop and commercialize SIM0278 overseas for a total milestone payment of $492 million. Simcere Pharmaceutical is also moving to explore the indications of SIM0278 overseas, and the partners are actively preparing for clinical studies in the U.S.
The first-in-human trial of SIM0278 marks an important milestone on its journey to benefit patients around the world. Simcere Pharmaceutical will continue to work with partners to promote the clinical validation of SIM0278 globally and look forward to bringing new treatment options to patients with autoimmune diseases around the world as soon as possible.
REFERENCES
1. Stander, S., Atopic Dermatitis. N Engl J Med, 2021. 384(12): p. 1136-1143.
2. Chinese Journal of Dermatology 2020, 53(2):81-88
3. Seegraber M, Srour J, et al. Expert Rev Clin Pharmacol 2018, 11:467-74.
4. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf.
5. Upadacitinib label https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
6. Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol,2003,74(6):961-965