On April 29, 2024, Simcere Pharmaceutical(2096.HK) and AnDiCon Biotech Co., Ltd. (hereinafter referred to as "AnDiCon") announced that the Phase III clinical trial of their anti-influenza innovative drug, Deunoxavir Marboxil Tablets (ADC189), has achieved its primary endpoint with significant efficacy and safety results. Deunoxavir Marboxil Tablets is a novel treatment for influenza A and B in adults and adolescents, requiring only one oral dose to inhibit the influenza virus replication within 24 hours.
The study, led by Ruijin Hospital Shanghai Jiaotong University School Of Medicine, is a multi-center, randomized, double-blind, placebo-controlled Phase II/III clinical study involving over 600 uncomplicated adolescent and adult patients with acute influenza. The trial was conducted at 73 centers across China. The primary endpoint was the time to remission of seven influenza symptoms: cough, sore throat, headache, nasal congestion, fever or chills, muscle or joint pain, and fatigue.
The results showed a significant improvement of 26.543% in median time to remission for patients treated with Deunoxavir Marboxil Tablets compared to the placebo group (p<0.0001). Further, the safety evaluation demonstrated that Deunoxavir Marboxil Tablets had a favorable safety profile and were well tolerated by patients, with fewer adverse events related to the study drug in the treatment group than in the placebo group.
Based on the mechanism of action of Deunoxavir Marboxil Tablets and the data from the phase II/III clinical study, several potential clinical therapeutic advantages has been identified:
One-tablet "cure": Unlike Oseltamivir, which requires five consecutive days of treatment, the entire oral dose of Deunoxavir Marboxil is contained within a single tablet.
One-day "turnaround": The tablets efficiently halt influenza virus replication within 24 hours after administration.
Mechanism of action diagram
These clinical trial findings highlight Deunoxavir Marboxil Tablets' potential to revolutionize the treatment of influenza, offering significant benefits in terms of ease-of-use and rapid onset of action. Simcere Pharmaceutical Group and AnDiCon look forward to continuing their collaboration to bring this innovative medication to market as soon as possible.
About Deunoxavir Marboxil Tablets
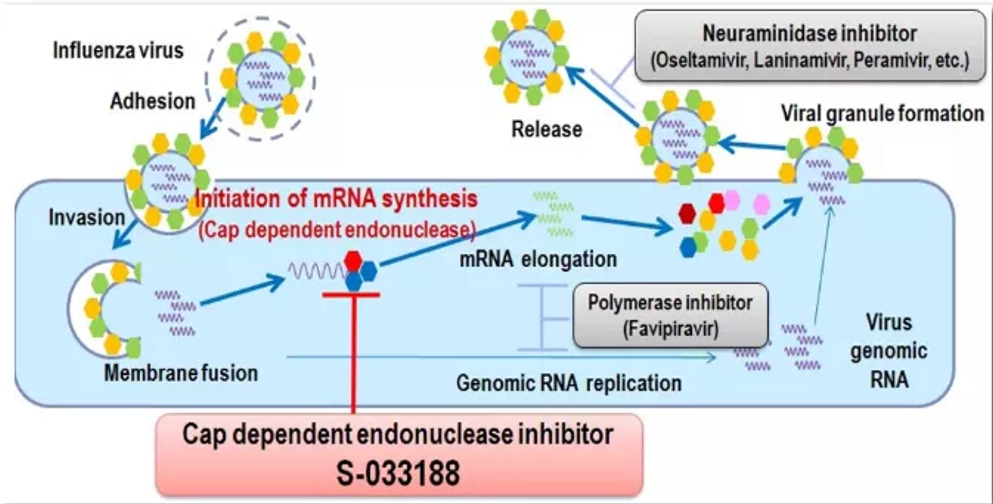
Deunoxavir Marboxil Tablets (ADC189) is a cap-dependent nucleic acid endonuclease inhibitor. The Tablets inhibit the cap-dependent nucleic acid endonuclease in influenza viruses, preventing their transcriptin, and, consequently, rendering them incapable of replicating. This potent action enables Deunoxavir Marboxil Tablets to combat influenza viruses from the root cause.
Clinical research data have shown that Deunoxavir Marboxil Tablets exhibit significant antiviral activity against avian influenza A, B and HPAI viruses, Additionally, they offer several advantages, including oral efficacy independent of food intake and an overall favorable safety profile.
About AnDiCon
AnDiCon is a biopharmaceutical high-tech company focusing on the development and industrialization of new respiratory anti-infective drugs, with a team led by a number of top scientists and academicians in China. Relying on the advantages of its DMPK new drug discovery platform, AnDiCon's innovative product pipeline covers the domain of respiratory anti-infection and gynecology, including influenza, respiratory syncytial virus, oral drugs for endometriosis, and uterine fibroids.