On Jul. 11, 2024, Simcere Pharmaceutical Co., Ltd. (2096.HK) announced that XIANNUOXIN® (simnotrelvir tablets/ritonavir tablets (co-packaged)) has been granted regular approval by the National Medical Products Administration (NMPA), transitioning from conditional approval, for the treatment of mild to moderate COVID-19 infections in adult patients. XIANNUOXIN® has become the first oral anti-COVID-19 drug obtaining regular approval in China.
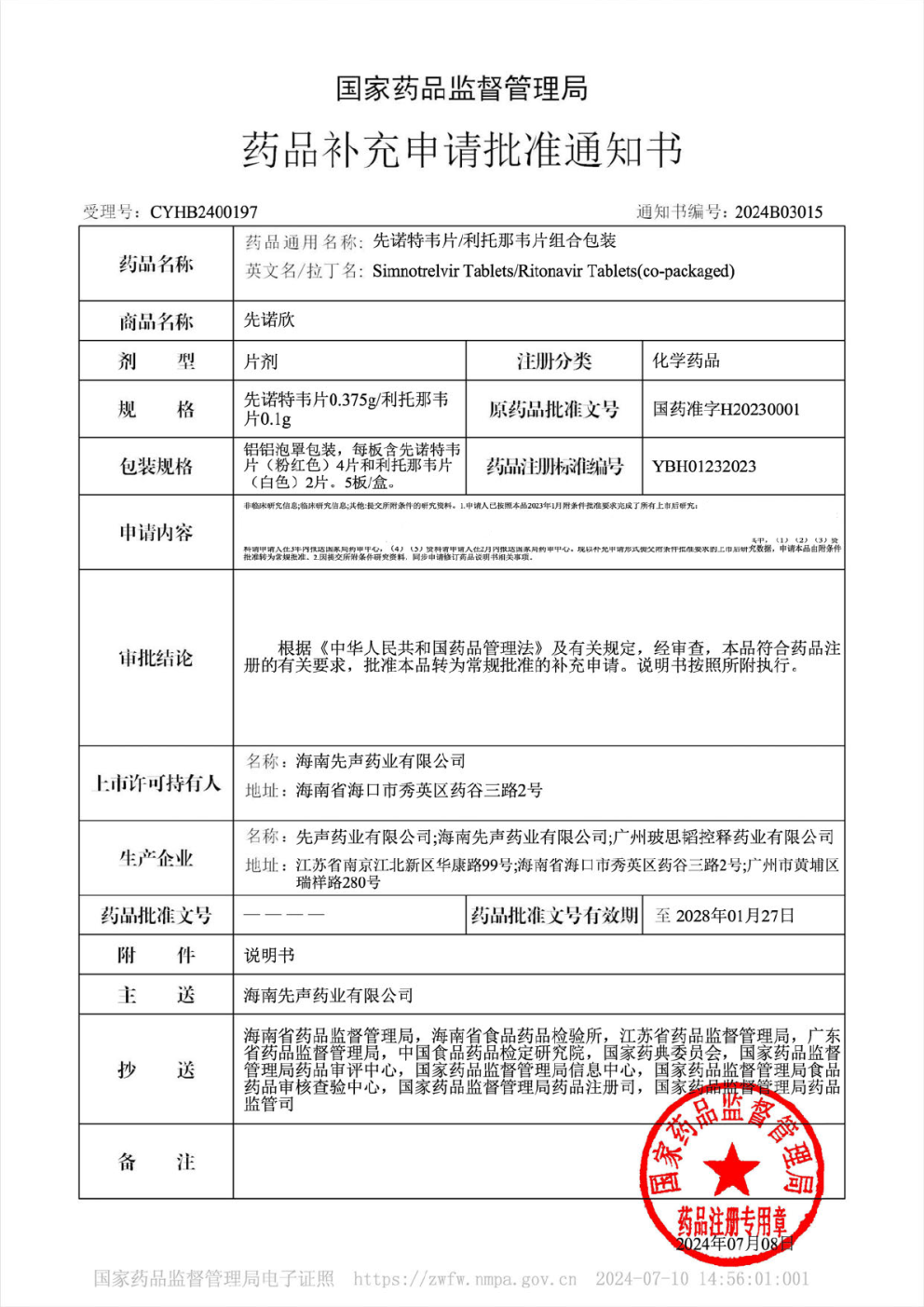
Notice on regular approval of XIANNUOXIN®
Rapid viral clearance and potent antiviral efficacy; clinical research earning global recognition
XIANNUOXIN® offers three major benefits: rapid and substantial reduction of viral load, a shortened course of disease and time to get a negative result for patients infected with COVID-19, and more reliable 3CL target. Phase II/III clinical studies demonstrated that XIANNUOXIN® rapidly and substantially decreased viral load by more than 96% while maintaining excellent safety and tolerability. On Jan. 28, 2023, XIANNUOXIN® was approved for conditional marketing by the NMPA.
The preclinical, Phase I, and Phase Ib studies of XIANNUOXIN® were successively published in 2023 in the sub-journals of Nature, the European Journal of Pharmaceutical Sciences, and the sub-journals of the Lancet. The Phase II/III clinical study was published in the New England Journal of Medicine in Jan. 2024. The study results have garnered international academic recognition and robustly demonstrated the efficacy and safety of XIANNUOXIN® across a broad spectrum of patients with mild to moderate COVID-19 infections. Consequently, XIANNUOXIN® has emerged as the pioneering anti-COVID-19 drug targeting 3CL, backed by a comprehensive body of evidence in China.
Large-scale real-world study to demonstrate efficacy and safety
After the conditional marketing of XIANNUOXIN®, the first large-scale real-world study on marketed anti-COVID-19 drugs in China was initiated in May 2023. The study, titled "Study on Therapeutic Effect of Anti-COVID-19 Drugs on COVID-19 Infections in Chinese Medical and Health Institutions," was led by the Principal Investigator Professor Jiang Rongmeng, Vice President of Beijing Ditan Hospital Capital Medical University.
This study was conducted in collaboration with several tertiary hospitals nationwide and in partnership with doctors from various primary healthcare institutions, where specialized digital tools were employed to enroll and follow up on patients with mild to moderate COVID-19 infections and collect treatment outcomes, involving 42 institutions and over 3300 patients.
The study findings demonstrated that XIANNUOXIN® achieved significant improvements in reducing the risk of hospitalization, decreasing the usage of other symptom relief medications, shortening the time to symptom recovery, and accelerating viral clearance. These results further validated the efficacy and safety of XIANNUOXIN®, establishing its wide applicability in medical practice.
XIANNUOXIN® packaging
As a product in the National Reimbursement Drug List, improved accessibility is expected
The accessibility of XIANNUOXIN® has been consistently enhanced since its marketing. XIANNUOXIN® was included in the temporary COVID-19 special medication insurance after the conditional approval. In Dec. 2023, it was officially incorporated into the National Reimbursement Drug List. The cost per box/course dropped to RMB 479, just a quarter of the price of comparable imported drugs, and patients now only bear an out-of-pocket expense of less than RMB 100 after insurance reimbursement.
As of now, XIANNUOXIN® has been adopted by over 3500 medical institutions nationwide, benefiting approximately 900,000 patients. Following this full approval, patients will soon have easier access to the medication through a variety of channels, which ensures prompt antiviral treatment within the critical 72-hour window for COVID-19 infection, thereby shortening the duration of the illness, alleviating the symptoms, and reducing the risk of critically ill cases.
About XIANNUOXIN®
XIANNUOXIN® (simnotrelvir tablets/ritonavir tablets (co-packaged)) is an oral small molecule anti-COVID-19 innovative drug. Simnotrelvir targeting the 3CL protease necessary for the replication of SARS-CoV-2 in combination with low-dose ritonavir helps to slow down the metabolism and clearance of simnotrelvir in the body and improve the antiviral effect.
XIANNUOXIN®, jointly developed by Simcere Pharmaceutical Co., Ltd., Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and Wuhan Institute of Virology, is China's first independently developed 3CL-targeting oral anti-COVID-19 innovative drug with proprietary intellectual property rights.